FTC Enforcement: What It Means for Your Medications and Supplements
When you buy a medication or supplement, you expect the label to tell the truth. That’s where FTC enforcement, the Federal Trade Commission’s authority to stop deceptive marketing in the U.S. pharmaceutical and supplement markets. It’s not just about ads—it’s about keeping dangerous lies off store shelves and out of your medicine cabinet. The FTC doesn’t just fine companies. It forces them to stop lying, recall products, and pay back consumers. And when it comes to drugs and supplements, those lies can cost lives.
Take generic drugs, lower-cost versions of brand-name medications that must meet the same FDA standards for safety and effectiveness. Some companies try to trick people into thinking their generic is inferior, or worse, that it’s unsafe. The FTC steps in when ads suggest generics don’t work as well—because that’s false. They also go after companies selling supplements that claim to cure cancer, reverse aging, or replace prescription drugs. These aren’t harmless claims. People delay real treatment because they believe the hype. The FTC has shut down dozens of these operations in the last five years, often working with the FDA to pull products off the market. You’ll see FTC actions linked to posts about drug safety, how side effects are tracked, reported, and acted on by regulators and patients alike—like when someone reports a rare reaction to a generic thyroid med, or when a supplement falsely claims to boost immunity. The FTC doesn’t investigate every complaint, but it does act when patterns emerge. Your report matters. That’s why posts on how to file a MedWatch report or appeal a denied generic prescription tie directly into this system.
And then there’s supplement regulation, the loose framework that lets companies sell vitamins, herbs, and weight-loss pills without proving they work. The FTC fills the gap. If a company says their protein powder builds muscle like steroids, or their sleep aid works better than melatonin, the FTC can demand proof—or shut them down. That’s why you’ll find posts here about misleading claims on labels, fake testimonials, and how to spot a scam. The FTC doesn’t regulate ingredients, but it does regulate what’s said about them. That’s your shield.
What you’ll find in the posts below isn’t just a list of articles—it’s a map of how FTC enforcement touches your daily health choices. From how generic substitution rules are enforced, to why your insurance won’t cover a certain brand, to how false claims about DOACs or biosimilars get challenged, these stories show the real-world impact of a government agency most people never think about. You don’t need to understand legal jargon to benefit. You just need to know: if it sounds too good to be true, the FTC might already be looking.
 3 December 2025
3 December 2025
Antitrust Issues in Generic Substitution: How Big Pharma Blocks Cheaper Drugs
Big pharma is using legal loopholes to block cheaper generic drugs by pulling older versions off the market. Learn how product hopping, REMS abuse, and court rulings are affecting drug prices and what’s being done to stop it.
Latest Posts
-

How to Check Lot Numbers and Recalls When Clearing Expired Medications
-

Cefaclor for Strep Throat: Uses, Dosing, Safety, and Alternatives (2025 Guide)
-
The Impact of Conjugated Estrogens USP on Mental Health
-

Alfacalcidol and Skin Health: What You Need to Know
-
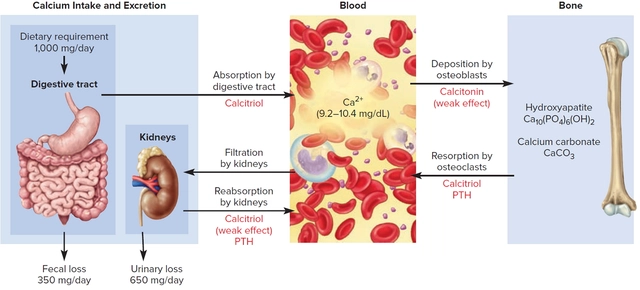
Lansoprazole and its impact on vitamin and mineral absorption

11