Bioequivalence Study: What It Means for Generic Drugs and Your Health
When you switch from a brand-name pill to a cheaper generic, you’re trusting that it does the same job. That trust comes from a bioequivalence study, a scientific test that proves a generic drug releases the same amount of active ingredient into your bloodstream at the same rate as the original. Also known as bioavailability study, it’s the gatekeeper between low-cost meds and real patient safety. Without this step, a generic could be too weak to work—or too strong and cause side effects.
These studies don’t just compare pills. They track how your body absorbs the drug—how fast it hits your blood, how high it goes, and how long it lasts. For drugs like blood thinners, thyroid meds, or seizure treatments, even small differences can throw off your whole treatment. That’s why the FDA, the U.S. agency that sets strict standards for drug approval requires bioequivalence data before any generic hits the shelf. But not all countries have the same rules. In the European Union, a region with its own regulatory system for generic medicines, approval paths vary by country, and some generics enter the market with less scrutiny. That’s why you might notice different results when switching brands or manufacturers—even if both are labeled "generic."
What you won’t see in the bottle? The name of the company that ran the bioequivalence study. Big pharma sometimes delays generics by tweaking the original drug just enough to dodge competition—a trick called "product hopping." Meanwhile, patients are left guessing why their new pill feels different. A medication journal, a simple log of how you feel after each dose can help you spot patterns: Is your energy down? Are you getting more side effects? That info isn’t just personal—it’s data that can trigger a safety review.
It’s not just about the drug itself. Factors like fillers, coatings, and how the pill breaks down in your stomach can change absorption. That’s why some people react differently to generics from different manufacturers. The science says they’re equivalent. But your body doesn’t always care what the study says—it cares what it feels. That’s why tracking your response matters more than ever, especially if you’re on meds where precision counts.
What you’ll find in the posts below? Real stories and hard facts about how bioequivalence studies shape what’s in your medicine cabinet. From how the EU handles generic approvals to why your insurance might deny a specific generic brand, we cover the hidden rules behind the pills you take. You’ll also see how switching to generics can save money—when done right—and when it might backfire. No fluff. Just what you need to know to stay safe, informed, and in control.
 1 December 2025
1 December 2025
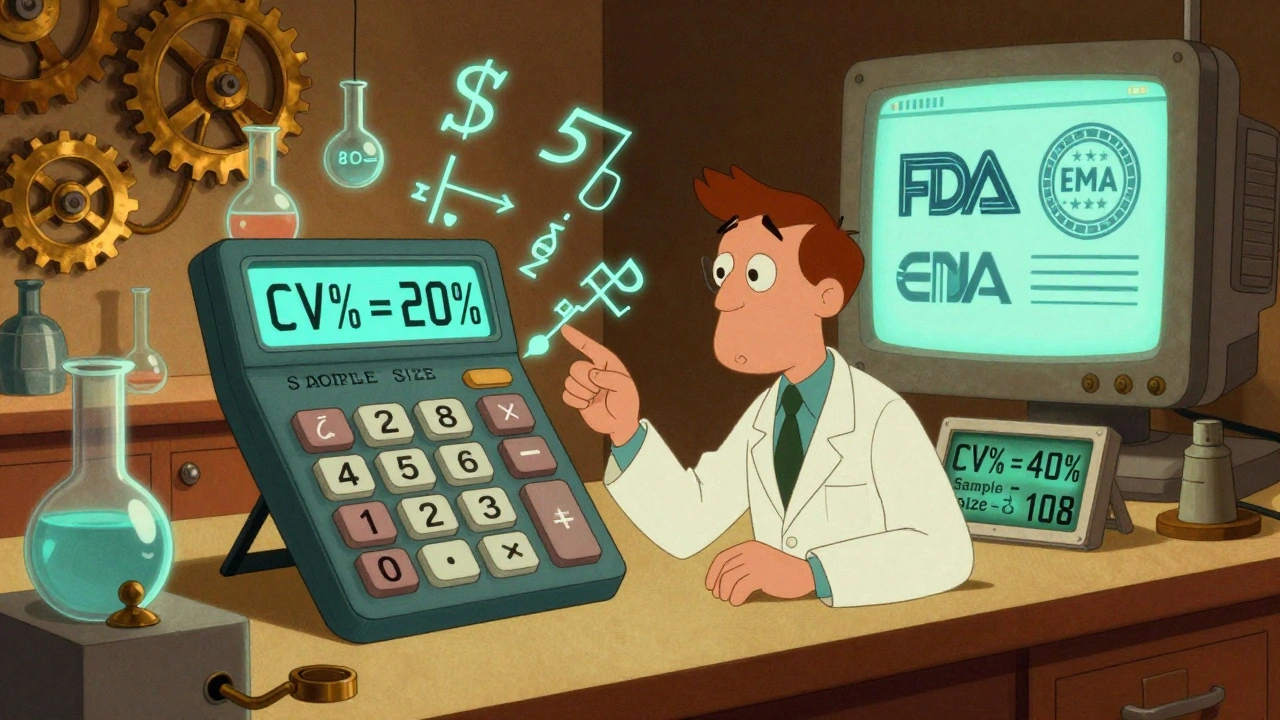
Statistical Analysis in BE Studies: How to Calculate Power and Sample Size Correctly
Learn how to correctly calculate power and sample size for bioequivalence studies to avoid costly failures. Understand CV%, GMR, regulatory requirements, and tools used by industry professionals.
Latest Posts
-
The Link Between Hepatic Encephalopathy and Sleep Disturbances
-

Boost Your Overall Health with the Power of Fructo-Oligosaccharides Dietary Supplement
-
SNRI Medications and Side Effects: Venlafaxine, Duloxetine, and Others
-
Depression Management: Medications, Therapy, and Lifestyle Changes That Work
-

Online Pharmacy Counterfeits: The Hidden Dangers of Buying Medicines Online

9