BE Study Design: What You Need to Know About Bioequivalence and Generic Drug Testing
When you switch from a brand-name drug to a generic, you’re trusting that it will work the same way. That trust comes from BE study design, a standardized testing method used to prove that a generic drug releases the same amount of active ingredient into the body at the same rate as the original. Also known as bioequivalence study, it’s the backbone of every generic drug approval in the U.S., EU, and most other countries. Without it, there’s no way to know if that cheaper pill will treat your condition just as well—or if it might cause unexpected side effects.
BE study design isn’t just about matching doses. It’s about tracking how your body absorbs, processes, and eliminates the drug. This is called pharmacokinetics, the science of how drugs move through the body over time. Researchers give volunteers both the brand and generic versions, then take frequent blood samples to measure drug levels. If the generic’s levels stay within 80% to 125% of the brand’s across all key measurements, it’s considered bioequivalent. That’s the legal threshold—and it’s not just a formality. For drugs like warfarin, levothyroxine, or seizure medications, even small differences can mean the difference between control and crisis.
But BE study design doesn’t just protect patients. It also shapes the market. Big pharma sometimes delays generics by changing the drug’s formulation just enough to force new BE studies, a tactic called product hopping. Meanwhile, regulators in the EU and U.S. are tightening rules to close loopholes that let inferior generics slip through. That’s why some generics work perfectly, while others cause complaints. The difference often comes down to how strictly the BE study was done—and who paid for it.
What you’ll find in these articles are real-world stories about BE study design in action: how it affects your prescriptions, why some generics trigger side effects, and how insurance denials sometimes come down to whether a drug passed bioequivalence testing. You’ll see how manufacturers handle drug substitutions, how regulatory bodies enforce standards, and why a pill that looks identical might not act the same inside your body. This isn’t theory. It’s the hidden science behind every generic you take—and the reason some people feel worse after switching.
 1 December 2025
1 December 2025
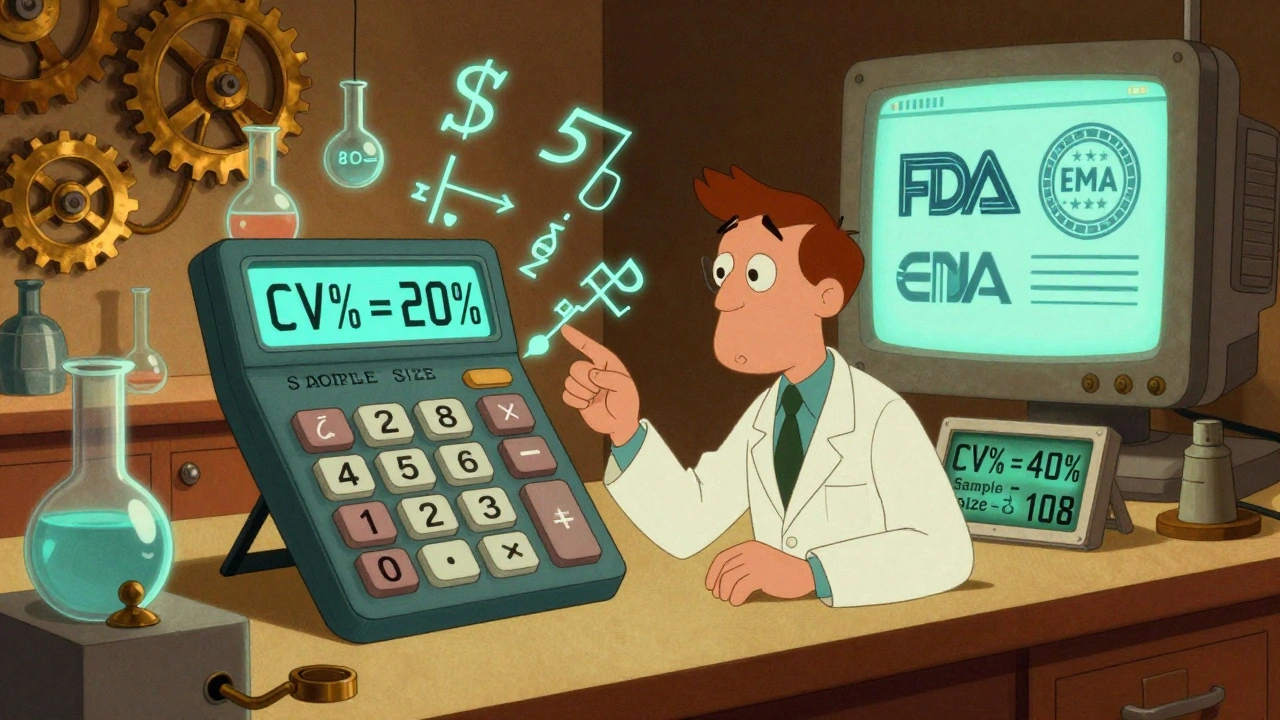
Statistical Analysis in BE Studies: How to Calculate Power and Sample Size Correctly
Learn how to correctly calculate power and sample size for bioequivalence studies to avoid costly failures. Understand CV%, GMR, regulatory requirements, and tools used by industry professionals.
Latest Posts
-

PCSK9 Inhibitors vs Statins: Side Effects and Outcomes
-

Cochlear Implants: Top Benefits for Severe Hearing Loss
-
Iverheal (Ivermectin) vs Top Antiparasitic Alternatives - Detailed Comparison
-

Cheap Generic Tetracycline Online - Safe Ways to Save on Antibiotics
-
Antihistamine Allergies and Cross-Reactivity: What to Watch For

9