Biosimilar vs Generic: What’s the Real Difference?
When you hear generic medication, a chemically identical copy of a brand-name drug that becomes available after the patent expires. Also known as brand-name equivalent, it is a straightforward, low-cost alternative that works the same way in your body. you think it’s the same as a biosimilar, a highly similar version of a complex biologic drug, made from living cells rather than chemicals. Also known as follow-on biologic, it’s not a copy in the traditional sense—it’s a close match, but not identical. That’s where people get confused. They’re both cheaper than the original, but they come from totally different worlds. A generic is like cloning a Lego brick—you take the exact blueprint and rebuild it. A biosimilar is more like recreating a handmade wooden chair using the same type of wood and tools, but no two will be perfectly alike because wood grows differently each time.
Why does this matter? Because biologic drugs, medications made from living organisms like cells or proteins, used for conditions like rheumatoid arthritis, cancer, or diabetes. Also known as biopharmaceuticals, they are complex molecules that can’t be replicated exactly like a pill. aren’t made in a lab with chemicals—they’re grown in living systems. Think insulin made by bacteria, or antibodies produced in cell cultures. That’s why you can’t just swap one biosimilar for another like you would with generic aspirin. The FDA requires biosimilars to show they work the same way and have no meaningful difference in safety or effectiveness—but they still have slight variations in structure. Generics? They have to be chemically identical down to the last atom. That’s why a generic metformin pill from any brand will act exactly like the brand-name version. But a biosimilar for Humira might have tiny differences in how it binds to cells, which is why doctors sometimes stick with the original unless you’re switching for cost reasons.
You’ll see both in your prescriptions, but they’re used for different things. Generics are everywhere—blood pressure pills, antidepressants, antibiotics. Biosimilars are for serious, chronic conditions where biologics are the only option. If you’re on a drug like Enbrel or Rituxan, you might be offered a biosimilar to cut costs. But if you’re on metformin or lisinopril, you’re almost certainly taking a generic. And here’s the thing: insurance companies push generics hard because they’re cheap and proven. They’re starting to push biosimilars too, but the rules are different. You can’t just swap a biosimilar without your doctor’s okay. You can swap a generic without even telling them.
That’s why it’s so important to know what you’re getting. If your pharmacy switches your drug without telling you, ask: Is this a generic? Or a biosimilar? One change is routine. The other needs a conversation. You’ll find real stories below—people who switched, what happened, and how they made sure they stayed safe. Whether you’re saving money or just trying to understand your treatment, knowing the difference between these two types of affordable drugs isn’t just helpful—it’s essential.
 20 November 2025
20 November 2025
Interchangeability: When Biosimilars Can Be Substituted Automatically
Learn when and how biosimilars can be automatically substituted for biologic drugs in the U.S., including FDA rules, state laws, patient risks, and cost savings. Understand the difference between interchangeable and regular biosimilars.
Latest Posts
-
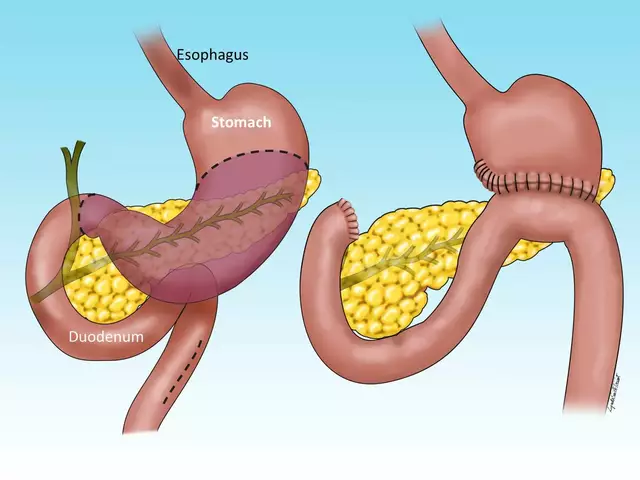
The Importance of Regular Checkups for Atrophic Gastroenteritis Patients
-
MS Relapse vs. Pseudorelapse: How to Tell the Difference and Why Steroids May Not Be the Answer
-

Keeping a Medication Journal: Tracking Your Response to Generic Medications
-
Import Inspections: How the FDA Monitors Drugs Entering the US
-

Mysimba vs Weight‑Loss Alternatives: Full 2025 Comparison

8