When you take multiple generic medications, you might think you're just getting the same drugs at a lower price. But what you're not seeing-what’s hidden in the pills and liquids-is a hidden mix of chemicals that aren’t supposed to do anything. These are called inactive ingredients, or excipients. And when they stack up across several generics, they can cause real problems-even if each drug works fine on its own.
What Are Inactive Ingredients, Really?
Inactive ingredients are the non-drug parts of a pill or liquid. They help the medicine hold its shape, dissolve properly, taste better, or last longer on the shelf. Common ones include lactose (a milk sugar), propylene glycol (a solvent), food dyes like tartrazine, and preservatives like bisulfites. These aren’t meant to treat your condition. But they’re everywhere-in nearly every generic medication you take.
Here’s the catch: two generic versions of the same drug-say, metformin or levothyroxine-can have completely different inactive ingredients. The FDA only requires that the active ingredient match the brand-name version within 80-125% of its effect. Nothing about the fillers, binders, or dyes. So one brand might use corn starch and titanium dioxide, while another uses lactose and FD&C Red No. 40. And if you’re taking three different generics, you might be getting all three of those ingredients at once.
Why This Matters More Than You Think
Most people assume that if a pill works for them once, it’ll work every time. But that’s not always true. A 2020 study found that someone taking 10 prescription meds ingests about 2.8 grams of inactive ingredients per day. That’s more than half a teaspoon of non-medicinal chemicals-every single day.
For most, that’s harmless. But for some, it’s enough to trigger reactions. Lactose intolerance affects about 65% of the global population. If you’re on three different generics that each contain 75 mg of lactose, you’re hitting 225 mg daily. That’s below the typical 12g tolerance threshold-but for sensitive people, even 1-2 grams can cause bloating, cramps, or diarrhea. One Reddit user described how switching to three generic heart meds led to severe GI distress, even though each one was fine alone.
Then there are dyes. Tartrazine (Yellow No. 5) is in about 4% of oral meds. It’s linked to hives and asthma flare-ups in sensitive people. Bisulfites, used as preservatives in injectables and nebulizer solutions, can trigger life-threatening reactions in 5-10% of asthmatics. And these aren’t rare. They’re in dozens of common generics.
When the Same Ingredient Shows Up in Multiple Drugs
The real danger isn’t just one bad ingredient. It’s the cumulative exposure across multiple prescriptions.
Imagine someone with hypothyroidism on levothyroxine, high blood pressure on amlodipine, and diabetes on metformin-all generics. Each uses a different manufacturer. One uses lactose. Another uses propylene glycol. The third uses gelatin and titanium dioxide. None of those alone would bother most people. But together? They can overwhelm the body’s ability to process them, especially in older adults or those with kidney or liver issues.
There’s documented evidence. The FDA has recorded cases where patients on digoxin-a heart drug with a narrow safety window-saw their levels drop after switching generics. Why? Because the new version’s excipients changed how quickly the drug was absorbed. The active ingredient was still the same. But the delivery wasn’t.
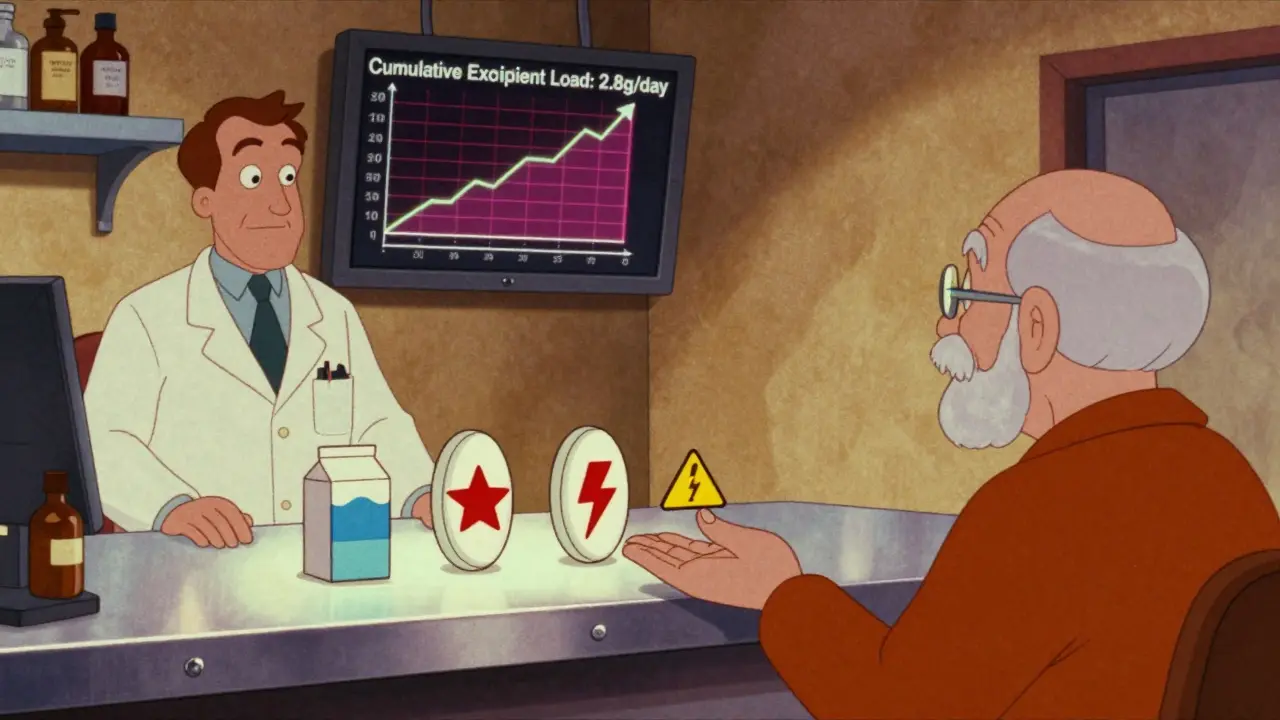
Brand vs. Generic: The Hidden Trade-Off
Brand-name drugs usually stick to the same inactive ingredients across batches. That consistency helps avoid surprises. Generics? They’re cheaper-often 80-85% less expensive-but they can change suppliers, formulas, or fillers without telling you. The European Medicines Agency openly admits that generics can differ in appearance, name, and excipients. The FDA says these differences are acceptable as long as they don’t affect how the drug works. But that’s not always true.
A 2022 FDA review found that some generic antiepileptic drugs had up to a 20% difference in peak blood concentration compared to the brand version-because of excipient changes. That’s not a small shift. For seizure patients, it could mean more seizures or more side effects.
And here’s the kicker: most patients don’t know their meds have changed. Pharmacists often switch generics automatically based on cost or availability. Unless you check the label or ask, you won’t know you’re now on a different formulation.
Who’s at Risk-and How to Spot It
You’re more likely to have problems if you:
- Take 4 or more medications daily (the average Medicare beneficiary takes 4.8)
- Have known allergies or intolerances (lactose, sulfites, dyes)
- Have chronic conditions like asthma, IBS, or autoimmune disorders
- Are elderly or have reduced liver/kidney function
Signs your meds might be clashing with their fillers:
- Unexplained GI upset (bloating, nausea, diarrhea) after starting a new generic
- Skin rashes or hives that appear after switching medications
- Medication seems less effective-even though you’re taking it exactly as prescribed
- Reactions happen only when you take multiple generics together
According to a 2022 survey by the National Community Pharmacists Association, 23% of pharmacists see at least one patient per month with suspected excipient-related reactions. The FDA’s Adverse Event Reporting System has over 1,200 reports from 2020-2023 linked to these issues-mostly GI distress, skin reactions, and reduced effectiveness.
What You Can Do
You don’t have to accept this as normal. Here’s how to protect yourself:
- Check your labels. Look at the Drug Facts or Patient Information leaflet. Inactive ingredients are listed there. Don’t just assume they’re the same.
- Ask your pharmacist. Say: “Are all these generics using the same fillers? Could any of them contain lactose, tartrazine, or bisulfites?”
- Use DailyMed or the FDA’s Inactive Ingredient Database. Type in the NDC code (found on the bottle) to see the exact ingredients in your version.
- Request consistency. If you’ve been on a generic that works, ask your pharmacist to stick with the same manufacturer. You’re allowed to do this.
- Track your symptoms. Keep a log: what meds you took, when you took them, and how you felt. Patterns matter.
One 2021 study in the American Journal of Health-System Pharmacy found that 78% of patients with suspected excipient reactions improved after switching to a different generic version with compatible fillers.
The Bigger Picture
The generic drug market is huge-$187.8 billion in 2022, and growing fast. As more people take more meds, especially older adults with multiple conditions, the chance of excipient interactions rises. By 2030, experts predict these issues could cost the U.S. healthcare system over $2.3 billion a year in extra ER visits, hospitalizations, and ineffective treatments.
Regulators are starting to wake up. The FDA launched its Inactive Ingredient Transparency Initiative in January 2024, requiring full digital labeling of all excipients by December 2025. The European Union already requires manufacturers to justify using known allergens in generics. And tools like MedCheck AI, released in late 2023, can scan your prescription list and flag risky combinations with 89.7% accuracy.
But until those systems are fully in place, the responsibility falls on you and your pharmacist. Because while the active ingredients get all the attention, it’s the silent players-the fillers, dyes, and preservatives-that can turn a safe regimen into a health risk.
Final Thought
Generic drugs saved billions in healthcare costs. That’s good. But assuming they’re all identical? That’s dangerous. The same pill, made by a different company, can behave differently-not because of the drug inside, but because of what’s around it. Your body doesn’t care about the brand name. It reacts to what’s in the pill. And if you’re taking multiple generics, you’re playing a game of chemical roulette. Don’t leave it to chance.
Can inactive ingredients in generic drugs cause allergic reactions?
Yes. Common inactive ingredients like lactose, tartrazine (Yellow No. 5), bisulfites, and certain dyes can trigger allergic or intolerance reactions. These aren’t drug allergies-they’re reactions to the fillers. Symptoms include hives, swelling, asthma flare-ups, stomach cramps, or diarrhea. People with known sensitivities to these substances are at higher risk, especially when taking multiple generics that contain the same ingredient.
How do I know if my generic medication contains lactose?
Check the Drug Facts label or Patient Information leaflet that comes with your prescription. Lactose will be listed under "Inactive Ingredients." You can also search the NDC code (on the bottle) in the FDA’s Inactive Ingredient Database or use DailyMed.gov. If you’re unsure, ask your pharmacist to confirm the formulation.
Are brand-name drugs safer than generics because of inactive ingredients?
Brand-name drugs usually have more consistent inactive ingredient profiles across batches, which reduces the chance of unexpected reactions. Generics can change manufacturers and excipients without notice. While both are required to be bioequivalent in terms of active ingredients, generics are not required to match the fillers of the brand version. So if you’ve had a good experience with one brand, switching to a generic might introduce new, unseen risks.
Can switching between generic versions make my medication less effective?
Yes. In rare cases, changes in inactive ingredients can affect how quickly or completely a drug is absorbed. This is especially critical for medications with a narrow therapeutic index-like digoxin, levothyroxine, or certain antiseizure drugs. A 2022 FDA review found some generics showed up to 20% differences in peak blood levels compared to brand versions due to excipient changes. If your condition worsens after switching generics, talk to your doctor and pharmacist.
What should I do if I suspect my meds are causing side effects because of inactive ingredients?
Start by reviewing all your medications’ inactive ingredients. Look for common culprits: lactose, propylene glycol, tartrazine, bisulfites. Track when symptoms started and whether they began after switching generics. Talk to your pharmacist-they can help identify matching formulations or alternatives. If symptoms persist, report them to the FDA’s MedWatch program. Many cases have been resolved simply by switching to a different generic version with safer fillers.





Marlon Mentolaroc
January 23, 2026 AT 22:39Okay but let’s be real - if you’re taking 10 meds a day, you’ve already lost the game. The real issue isn’t lactose in your metformin, it’s that your doctor prescribed you 10 pills like it’s a buffet. I’ve seen patients on 14 different generics and then act shocked when they feel like garbage. The excipients? Minor. The polypharmacy? Catastrophic.
Marie-Pier D.
January 24, 2026 AT 08:55Thank you for posting this 💙 I’m a pharmacist in Ontario and I see this every week. One lady came in crying because her thyroid meds suddenly made her dizzy - switched from one generic to another, same active ingredient, different filler. We found lactose in the new one. She’s lactose intolerant. She didn’t even know that was possible. You’re not crazy if your meds feel different. It’s not all in your head.
Gina Beard
January 25, 2026 AT 15:20It’s not about the pills. It’s about the system that treats bodies like interchangeable parts.
Alexandra Enns
January 26, 2026 AT 02:48Oh wow. So now we’re blaming Big Pharma for not making generic drugs with the same fillers? 🤡 Canada’s healthcare system is already broken, but you want us to pay more for brand-name pills because some people can’t handle a little lactose? Newsflash - most of the world survives on cheaper meds. If you’re allergic to fillers, maybe don’t take 7 drugs at once. Also, FDA’s not hiding anything. It’s all on the label. You just don’t read it. 🙄
Juan Reibelo
January 26, 2026 AT 12:51My mom’s on 6 generics. She’s 72. Last month she started having cramps after switching her blood pressure med. We checked the label - new version had gelatin AND lactose. Old one had neither. She’s fine now that we switched back. It’s not paranoia. It’s pharmacology. And pharmacists? Most of them don’t even check unless you ask. You have to be your own advocate. Don’t let anyone tell you it’s ‘just a filler.’
Phil Maxwell
January 27, 2026 AT 20:02I didn’t even know this was a thing until my cousin had a rash after switching her antidepressant. We looked it up - FD&C Yellow No. 5. She’s never had allergies before. But now she avoids any generic with ‘tartrazine’ on the label. I just wish more people knew this stuff. It’s wild that something so small can mess you up.
Don Foster
January 28, 2026 AT 09:27Yall are overreacting to excipients like they’re toxins from a sci-fi movie. The body handles way worse stuff daily. You eat processed food with 20 additives, drink soda with phosphoric acid, breathe urban air - but now you’re crying because your metformin has corn starch? Wake up. The real problem is you’re too lazy to read the label. The FDA doesn’t lie. You just don’t look.
asa MNG
January 29, 2026 AT 12:26bro i just found out my anxiety med has lactose in it?? and im lactose intolerant?? 😭 i’ve been having stomach issues for 6 months and thought it was stress… i just switched back to brand name and i feel like a new person. also why does no one talk about this?? like why is this not common knowledge?? 😩
Himanshu Singh
January 29, 2026 AT 23:59This is a beautiful reminder that medicine is not just chemistry - it’s biology, culture, and individuality. Each body is a unique ecosystem. What’s harmless to one may be a storm to another. The system prioritizes cost over care, but awareness is the first step toward healing. Keep asking questions. Your body is listening.
Jamie Hooper
January 30, 2026 AT 01:53so like… i took 3 generics last week and my face broke out like i was 14 again?? i thought it was my shampoo. turns out one had red dye 40. i googled it. it’s in like 12% of pills?? why is this not on the front of the bottle?? like why??
Husain Atther
January 30, 2026 AT 20:43While the concerns raised are valid, it is important to balance them with the broader context of healthcare accessibility. Generic medications enable millions to afford life-saving treatments. The solution lies not in rejecting generics, but in improving transparency and education. Regulatory bodies are moving in the right direction, and informed patients are the key to progress.
Shelby Marcel
January 31, 2026 AT 11:24wait so if i switch from one generic to another and feel weird… it might not be my body going haywire but the filler?? like… how do i even know which one has what??